Evidence based
Comprehensive List of CBD Research Studies
CBD is one of the most well-researched compounds in the natural world.
There’s been a lot of research published on the health benefits of cannabis and CBD products over the last few decades — but not all research is created equal.
There’s a big difference between cell culture studies compared to large-scale, double-blind clinical trials involving real humans.
Now that we’re in a new era of hemp and cannabis legalization, research efforts are ramping up to fill in the gaps in what we know — and just as importantly, what we don’t know about the health effects of cannabinoids.
Exhaustive List of Cannabidiol Research
To date, there have been well over 15,000 individual studies involving CBD.
Most of these studies are molecular analysis and in vitro research — which offer limited evidence for the benefits of CBD.
More recently, there’s been an aggressive push towards more high-quality in vivo and clinical trial studies. These are the studies that are really moving the needle when it comes to our academic understanding of cannabinoids.
CBD is no longer stigmatized, and the early body or research has done enough to prove the safety of CBD. Now all eyes are on the future — what is CBD capable of?
Does CBD actually work? Is there enough evidence to back up the suggested health benefits of this infamous cannabinoid?

CBD Research: Addiction
Research supports the following effects of CBD for addiction:
- Regulates the function of dopamine in the brain
- Alleviates common symptoms of drug withdrawal syndromes
- CBD may reduce cravings for tobacco
List of research studies involving CBD & addiction:
- Cannabidiol as an intervention for addictive behaviors: a systematic review of the evidence. (2015)
- Early Phase in the Development of Cannabidiol as a Treatment for Addiction: Opioid Relapse Takes Initial Center Stage. (2015)
- Cannabidiol reduces cigarette consumption in tobacco smokers: preliminary findings. (2013)
- Cannabidiol inhibitory effect on marble-burying behaviour: involvement of CB1 receptors. (2010)
- Cannabidiol is an allosteric modulator at mu-and delta-opioid receptors. (2006)
CBD Research: Anxiety
Research supports the following effects of CBD for anxiety:
- Boosts GABA function in the brain
- Regulates serotonin & dopamine activity
- Relaxes tight or tense muscles
- Alleviates inflammation in the brain
- Promotes a more restorative sleep
- Reduces cortisol levels
List of research studies involving CBD & anxiety:
- Study: Cannabidiol Holds Promise for Treating Psychosis (2020)
- Cannabidiol in anxiety and sleep: a large case series. (2019)
- Cannabidiol modulates serotonergic transmission and reverses both allodynia and anxiety-like behavior in a model of neuropathic pain. (2019)
- Cannabidiol in Anxiety and Sleep: A Large Case Series. (2019)
- Cannabidiol as a therapeutic alternative for post-traumatic stress disorder: From bench research to confirmation in human trials. (2018)
- Cannabidiolic acid methyl ester, a stable synthetic analogue of cannabidiolic acid, can produce 5‐HT1A receptor‐mediated suppression of nausea and anxiety in rats. (2018)
- Cannabidiol Regulation of Emotion and Emotional Memory Processing: Relevance for Treating Anxiety-related and Substance Abuse Disorders (2017)
- Cannabidiol Does Not Dampen Responses to Emotional Stimuli in Healthy Adults (2017)
- Effect of Prior Foot Shock Stress and Delta9-Tetrahydrocannabinol, Cannabidiolic Acid, and Cannabidiol on Anxiety-like Responding in the Light-dark Emergence Test in Rats (2017)
- Inverted U-Shaped Dose-Response Curve of the Anxiolytic Effect of Cannabidiol during Public Speaking in Real Life. (2017)
- Cannabidiol regulation of emotion and emotional memory processing: relevance for treating anxiety‐related and substance abuse disorders. (2017)
- Effects of Cannabidiol Oil for Pediatric Anxiety and Insomnia as Part of Posttraumatic Stress Disorder: A Case Report (2016)
- Cannabidiol as a Potential Treatment for Anxiety Disorders (2015)
- Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. (2015)
- Antidepressant-like and anxiolytic-like effects of cannabidiol: a chemical compound of Cannabis sativa. (2014)
- The Anxiolytic Effect of Cannabidiol on Chronically Stressed Mice Depends on Hippocampal Neurogenesis: Involvement of the Endocannabinoid System (2013)
- Does cannabidiol protect against adverse psychological effects of THC? (2013)
- Cannabidiol, a Cannabis Sativa Constituent, as an Anxiolytic Drug (2012)
- Neural basis of anxiolytic effects of cannabidiol (CBD) in generalized social anxiety disorder: a preliminary report. (2011)
- Cannabidiol reduces the anxiety induced by simulated public speaking in treatment-naive social phobia patients. (2011)
- Neural Basis of Anxiolytic Effects of Cannabidiol (CBD) in Generalized Social Anxiety Disorder: a Preliminary Report (2011)
- Cannabidiol Reduces the Anxiety Induced by Simulated Public Speaking in Treatment-naive Social Phobia Patients (2011)
- Distinct Effects of Δ9-Tetrahydrocannabinol and Cannabidiol on Neural Activation During Emotional Processing. (2009)
- Effects of cannabidiol (CBD) on regional cerebral blood flow. (2004)
- Effects of ipsapirone and cannabidiol on human experimental anxiety. (1993)
- Action of cannabidiol on the anxiety and other effects produced by Δ 9-THC in normal subjects. (1982)

CBD Research: Arthritis
Research supports the following effects of CBD for arthritis:
- Alleviates underlying inflammation
- Relieves chronic pain
- Prevents T-cell infiltration in rheumatoid arthritis
- Promotes recovery of the joint tissue
List of research studies involving CBD & arthritis:
- Pharmacokinetics, safety, and clinical efficacy of cannabidiol treatment in osteoarthritic dogs. (2018)
- Attenuation of early phase inflammation by cannabidiol prevents pain and nerve damage in rat osteoarthritis. (2017)
- Cannabidiol as an Emergent Therapeutic Strategy for Lessening the Impact of Inflammation on Oxidative Stress (2011)
- The Abnormal Cannabidiol Analogue O-1602 Reduces Nociception in a Rat Model of Acute Arthritis Via the Putative Cannabinoid Receptor GPR55 (2011)
- Oral Anti-inflammatory Activity of Cannabidiol, a Non-psychoactive Constituent of Cannabis, in Acute Carrageenan-induced Inflammation in the Rat Paw (2004)
- The nonpsychoactive cannabis constituent cannabidiol is an oral anti-arthritic therapeutic in murine collagen-induced arthritis. (2000)

CBD Research: Cancer
Research supports the following effects of CBD for cancer:
- Regulates key immune cells tasked with cancer detection
- May alleviate cancer-related pain
- Supports sleep duration and quality
- May boost hunger levels
- May slow tumor growth
- Alleviates nausea & vomiting associated with chemotherapy
- May support anxiety & depression associated with a cancer diagnosis
List of research studies involving CBD & cancer:
- Striking lung cancer response to self- administration of cannabidiol: A case report and literature review. (2019)
- Cannabidiol-induced apoptosis is mediated by activation of Noxa in human colorectal cancer cells. (2019)
- Concomitant Treatment of Malignant Brain Tumours With CBD – A Case Series and Review of the Literature. (2019)
- Report of Objective Clinical Responses of Cancer Patients to Pharmaceutical-grade Synthetic Cannabidiol. (2018)
- Cannabidiol rather than Cannabis sativa extracts inhibit cell growth and induce apoptosis in cervical cancer cells. (2016)
- Inhibition of Colon Carcinogenesis by a Standardized Cannabis Sativa Extract With High Content of Cannabidiol (2014)
- Cannabidiol as Potential Anticancer Drug (2013)
- Cannabidiol, a Non-psychoactive Cannabinoid Compound, Inhibits Proliferation and Invasion in U87-MG and T98G Glioma Cells Through a Multitarget Effect (2013)
- Triggering of the Trpv2 Channel by Cannabidiol Sensitizes Glioblastoma Cells to Cytotoxic Chemotherapeutic Agents (2013)
- An Open-label Extension Study to Investigate the Long-term Safety and Tolerability of THC/CBD Oromucosal Spray and Oromucosal Thc Spray in Patients With Terminal Cancer-related Pain Refractory to Strong Opioid Analgesics (2013)
- Non-THC cannabinoids inhibit prostate carcinoma growth in vitro and in vivo: pro-apoptotic effects and underlying mechanisms. (2013)
- COX-2 and PPAR-γ Confer Cannabidiol-Induced Apoptosis of Human Lung Cancer Cells. (2013)
- Cannabidiol as potential anticancer drug. (2013)
- Cannabidiol Inhibits Growth and Induces Programmed Cell Death in Kaposi Sarcoma-associated Herpesvirus-infected Endothelium (2012)
- Cannabidiol Inhibits Lung Cancer Cell Invasion and Metastasis Via Intercellular Adhesion Molecule-1 (2012)
- Cannabidiol Inhibits Growth and Induces Programmed Cell Death in Kaposi Sarcoma–Associated Herpesvirus-Infected Endothelium. (2012)
- Chemopreventive effect of the non-psychotropic phytocannabinoid cannabidiol on experimental colon cancer. (2012)
- Cannabidiol induces programmed cell death in breast cancer cells by coordinating the cross-talk between apoptosis and autophagy. (2011)
- Pathways mediating the effects of cannabidiol on the reduction of breast cancer cell proliferation, invasion, and metastasis. (2011)
- Cannabidiol Induces Programmed Cell Death in Breast Cancer Cells by Coordinating the Cross-talk between Apoptosis and Autophagy. (2011)
- Multicenter, double-blind, randomized, placebo-controlled, parallel-group study of the efficacy, safety, and tolerability of THC: CBD extract and THC extract in patients with intractable cancer-related pain. (2010)
- Cannabidiol enhances the inhibitory effects of Δ9-tetrahydrocannabinol on human glioblastoma cell proliferation and survival. (2010)
- A Comparative Study on Cannabidiol-induced Apoptosis in Murine Thymocytes and El-4 Thymoma Cells (2008)
- Cannabidiol as a Novel Inhibitor of Id-1 Gene Expression in Aggressive Breast Cancer Cells (2007)
- Antitumor activity of plant cannabinoids with emphasis on the effect of cannabidiol on human breast carcinoma. (2006)
- Cannabidiol-induced Apoptosis in Human Leukemia Cells: a Novel Role of Cannabidiol in the Regulation of P22phox and Nox4 Expression (2006)
- Gamma-irradiation Enhances Apoptosis Induced by Cannabidiol, a Non-psychotropic Cannabinoid, in Cultured Hl-60 Myeloblastic Leukemia Cells (2003)

CBD Research: Diabetes
Research supports the following effects of CBD for diabetes:
- Helps curb appetite & reduce weight
- Protects the liver
- Alleviates neuropathic pain
- Alleviates other side effects of diabetes
- Supports insulin function
- Supports faster wound healing
- Protects the insulin-secreting cells in the pancreas
List of research studies involving CBD & diabetes:
- The Atypical Cannabinoid Abn-CBD Reduces Inflammation and Protects Liver, Pancreas, and Adipose Tissue in a Mouse Model of Prediabetes and Non-alcoholic Fatty Liver Disease. (2020)
- Cannabidiol attenuates mechanical allodynia in streptozotocin-induced diabetic rats via serotonergic system activation through 5-HT1A receptors (2019)
- Efficacy and Safety of Cannabidiol and Tetrahydrocannabivarin on Glycemic and Lipid Parameters in Patients With Type 2 Diabetes: A Randomized, Double-Blind, Placebo-Controlled, Parallel Group Pilot Study (2016)
- Cannabidiol improves vasorelaxation in Zucker diabetic fatty rats through cyclooxygenase activation. (2014)
- Cannabidiol Protects Retinal Neurons by Preserving Glutamine Synthetase Activity in Diabetes (2010)
- Cannabidiol Attenuates Cardiac Dysfunction, Oxidative Stress, Fibrosis, and Inflammatory and Cell Death Signaling Pathways in Diabetic Cardiomyopathy (2010)
- Cannabidiol Arrests Onset of Autoimmune Diabetes in NOD Mice (2008)
- Cannabidiol Lowers Incidence of Diabetes in Non-obese Diabetic Mice (2006)
- Neuroprotective and Blood-retinal Barrier-preserving Effects of Cannabidiol in Experimental Diabetes (2006)

CBD Research: Epilepsy
Research supports the following effects of CBD for epilepsy:
- Alleviates convulsions
- Protects the nerve cells
- Modulates key receptors that regulate brain health
- Alleviates neuro-inflammation
- Supports GABA activity
List of research studies involving CBD & epilepsy:
- Anticonvulsive Properties of Cannabidiol in a Model of Generalized Seizure Are Transient Receptor Potential Vanilloid 1 Dependent. (2020)
- Epilepsy and cannabidiol: a guide to treatment. (2020)
- Cannabidiol in treatment of refractory epileptic spasms: An open-label study. (2020)
- The proposed mechanisms of action of CBD in epilepsy. (2020)
- Dose-Ranging Effect of Adjunctive Oral Cannabidiol vs Placebo on Convulsive Seizure Frequency in Dravet Syndrome. (2020)
- Cost-effectiveness of cannabinoids for pediatric drug-resistant epilepsy: protocol for a systematic review of economic evaluations. (2019)
- Update on Antiepileptic Drugs 2019 (2019)
- Randomized blinded controlled clinical trial to assess the effect of oral cannabidiol administration in addition to conventional antiepileptic treatment on seizure frequency in dogs with intractable idiopathic epilepsy. (2019)
- Food effect on pharmacokinetics of cannabidiol oral capsules in adult patients with refractory epilepsy (2019)
- Cannabis‐based products for pediatric epilepsy: A systematic review. (2019)
- Long‐term cannabidiol treatment in patients with Dravet syndrome: An open‐label extension trial. (2019)
- Cannabidiol in patients with Lennox‐Gastaut syndrome: Interim analysis of an open‐label extension study. (2019)
- Drug–drug interactions with cannabidiol (CBD) appear to have no effect on treatment response in an open-label Expanded Access Program. (2019)
- Randomized blinded controlled clinical trial to assess the effect of oral cannabidiol administration in addition to conventional antiepileptic treatment on seizure frequency in dogs with intractable idiopathic epilepsy. (2019)
- Long-Term Safety, Tolerability, and Efficacy of Cannabidiol in Children with Refractory Epilepsy: Results from an Expanded Access Program in the US. (2019)
- Pharmacological and Therapeutic Properties of Cannabidiol for Epilepsy. (2019)
- Quality of life in adults enrolled in an open-label study of cannabidiol (CBD) for treatment-resistant epilepsy. (2019)
- Potential clinical benefits of CBD-rich cannabis extracts over purified CBD in treatment-resistant epilepsy: observational data meta-analysis. (2018)
- An interaction between warfarin and cannabidiol, a case report. (2018)
- Cannabidiol in patients with seizures associated with Lennox-Gastaut syndrome (GWPCARE4): a randomised, double-blind, placebo-controlled phase 3 trial. (2018)
- Cannabidiol for Treatment of Childhood Epilepsy–A Cross-Sectional Survey. (2018)
- Effect of Cannabidiol on Drop Seizures in the Lennox–Gastaut Syndrome. (2018)
- Potential clinical benefits of CBD-rich Cannabis extracts over purified cannabidiol (CBD) in treatment-resistant epilepsy: observational data meta-analysis. (2018)
- Interactions between cannabidiol and commonly used antiepileptic drugs. (2017)
- Cannabidiol attenuates seizures and social deficits in a mouse model of Dravet syndrome. (2017)
- Historical perspective on the medical use of cannabis for epilepsy: Ancient times to the 1980s. (2017)
- Cannabinoids in the Treatment of Epilepsy: Hard Evidence at Last?. (2017)
- CBD-enriched medical cannabis for intractable pediatric epilepsy: the current Israeli experience. (2016)
- Cannabidiol in patients with treatment-resistant epilepsy: an open-label interventional trial. (2016)
- Cannabinoids and Epilepsy (2015)
- Drug–drug interaction between clobazam and cannabidiol in children with refractory epilepsy. (2015)
- Cannabinoids in the treatment of epilepsy. (2015)
- Nonpsychotropic plant cannabinoids, cannabidivarin (CBDV) and cannabidiol (CBD), activate and desensitize transient receptor potential vanilloid 1 (TRPV1) channels in vitro: potential for the treatment of neuronal hyperexcitability. (2014)
- The case for medical marijuana in epilepsy. (2014)
- Cannabidiol: pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. (2014)
- Cannabidiol displays antiepileptiform and antiseizure properties in vitro and in vivo. (2010)
- Downregulation of the CB1 cannabinoid receptor and related molecular elements of the endocannabinoid system in epileptic human hippocampus. (2008)
- Effects of cannabidiol on behavioral seizures caused by convulsant drugs or current in mice. (1982)
- Hypnotic and Antiepileptic Effects of Cannabidiol. (1981)

CBD Research: IBD
Research supports the following effects of CBD for IBD:
- Inhibits inflammation in the digestive tract
- Prevents mast cell activation
- Alleviates pain
- May support microbiome diversity
List of research studies involving CBD & IBD:
- A randomized, double-blind, placebo-controlled, parallel-group, pilot study of cannabidiol-rich botanical extract in the symptomatic treatment of ulcerative colitis. (2018)
- Cannabidiol in inflammatory bowel diseases: a brief overview. (2013)

CBD Research: Migraines & Headaches
Research supports the following effects of CBD for headaches:
- Relieves inflammation
- Blocks the transmission of pain
- May prevent the release of serotonin from platelets
- May reduce vascular spasms
List of research studies involving CBD & headaches:
- Cannabidiol, a non-psychotropic plant-derived cannabinoid, decreases inflammation in a murine model of acute lung injury: Role for the adenosine A2A receptor. (2012)
- Inhibition of an equilibrative nucleoside transporter by cannabidiol: a mechanism of cannabinoid immunosuppression. (2006)

CBD Research: Multiple Sclerosis
Research supports the following effects of CBD for multiple sclerosis:
- Resists the development of autoimmunity
- Reduces neuroinflammation
- Keeps T-cells from congregating around the nerves
- Reduces muscle spasms
- Supports bladder control
- Alleviates nerve pain
List of research studies involving CBD & MS:
- Daily Practice Managing Resistant Multiple Sclerosis Spasticity With Delta-9-Tetrahydrocannabinol: Cannabidiol Oromucosal Spray: A Systematic Review of Observational Studies (2019)
- Cannabidiol Attenuates Experimental Autoimmune Encephalomyelitis Model of Multiple Sclerosis Through Induction of Myeloid-Derived Suppressor Cells. (2018)
- Nabiximols (THC/CBD Oromucosal Spray, Sativex®) in Clinical Practice – Results of a Multicenter, Non-Interventional Study (MOVE 2) in Patients with Multiple Sclerosis Spasticity. (2014)
- A Double-blind, Randomized, Placebo-controlled, Parallel-group Study of THC/CBD Oromucosal Spray in Combination With the Existing Treatment Regimen, in the Relief of Central Neuropathic Pain in Patients With Multiple Sclerosis (2013)
- Cannabidiol provides long-lasting protection against the deleterious effects of inflammation in a viral model of multiple sclerosis: A role for A2A receptors. (2013)
- Cannabidiol provides long-lasting protection against the deleterious effects of inflammation in a viral model of multiple sclerosis: a role for A2A receptors. (2013)
- Symptomatic therapy in multiple sclerosis: the role of cannabinoids in treating spasticity. (2012)
- A placebo-controlled, parallel-group, randomized withdrawal study of subjects with symptoms of spasticity due to multiple sclerosis who are receiving long-term Sativex®(nabiximols). (2012)
- Cannabidiol inhibits pathogenic T cells, decreases spinal microglial activation and ameliorates multiple sclerosis‐like disease in C57BL/6 mice. (2011)
- Meta-analysis of the efficacy and safety of Sativex (nabiximols), on spasticity in people with multiple sclerosis. (2010)
- Cannabinoids in the management of spasticity associated with multiple sclerosis. (2008)
- Oromucosal Delta9-tetrahydrocannabinol/cannabidiol for Neuropathic Pain Associated With Multiple Sclerosis: an Uncontrolled, Open-label, 2-year Extension Trial (2007)
- Meta-analysis of Cannabis Based Treatments for Neuropathic and Multiple Sclerosis-related Pain (2007)
- Cannabinoid control of neuroinflammation related to multiple sclerosis. (2007)
- Sativex®: clinical efficacy and tolerability in the treatment of symptoms of multiple sclerosis and neuropathic pain. (2006)
- An open-label pilot study of cannabis-based extracts for bladder dysfunction in advanced multiple sclerosis. (2004)
- he cannabinoids: an overview. Therapeutic implications in vomiting and nausea after cancer chemotherapy, in appetite promotion, in multiple sclerosis and in neuroprotection. (2001)
- The perceived effects of smoked cannabis on patients with multiple sclerosis. (1997)

CBD Research: Nausea
Research supports the following effects of CBD for nausea:
- Increases anandamide levels in the medulla oblongata
- Alleviates inflammation
- Relaxes muscle cramping and pain
List of research studies involving CBD & nausea:
- Effect of Combined Oral Doses of Δ(9)-tetrahydrocannabinol (THC) and Cannabidiolic Acid (CBDA) on Acute and Anticipatory Nausea in Rat Models (2016)
- Cannabidiolic Acid Prevents Vomiting in Suncus Murinus and Nausea-induced Behaviour in Rats by Enhancing 5-HT1A Receptor Activation (2013)
- Suppression of Lithium Chloride-induced Conditioned Gaping (a Model of Nausea-induced Behaviour) in Rats (using the Taste Reactivity Test) With Metoclopramide Is Enhanced by Cannabidiolic Acid (2013)
- Cannabidiol, a Non-psychotropic Component of Cannabis, Attenuates Vomiting and Nausea-like Behaviour Via Indirect Agonism of 5-HT(1A) Somatodendritic Autoreceptors in the Dorsal Raphe Nucleus (2012)
- Interaction Between Non-psychotropic Cannabinoids in Marihuana: Effect of Cannabigerol (CBG) on the Anti-nausea or Anti-emetic Effects of Cannabidiol (CBD) in Rats and Shrews (2011)
- The Effects of Cannabidiol and Tetrahydrocannabinol on Motion-induced Emesis in Suncus Murinus (2008)
- Cannabidiol, a Non-psychoactive Component of Cannabis and Its Synthetic Dimethylheptyl Homolog Suppress Nausea in an Experimental Model With Rats (2002)
CBD Research: Pain
Research supports the following effects of CBD for pain:
- Reduces inflammation
- Alleviates common side-effects of pain
- Supports nerve cell health
- Activates pain receptors in the spinal cord and brain
List of research studies involving CBD & pain:
- Cannabidiol for pain treatment: focus on pharmacology and mechanism of action. (2020)
- Cannabidiol as adjunctive treatment of seizures associated with Lennox-Gastaut syndrome and Dravet syndrome (2019)
- Cannabidiol in anxiety and sleep: a large case series. (2019)
- Effects of cannabidiol in males and females in two different rat models of depression. (2019)
- Cannabidiol modulates serotonergic transmission and reverses both allodynia and anxiety-like behavior in a model of neuropathic pain. (2019)
- Evaluation of the effects of CBD hemp extract on opioid use and quality of life indicators in chronic pain patients: a prospective cohort study. (2019)
- Medicinal Properties of Cannabinoids, Terpenes, and Flavonoids in Cannabis, and Benefits in Migraine, Headache, and Pain: An Update on Current Evidence and Cannabis Science. (2018)
- Medical cannabis for neuropathic pain. (2018)
- Chronic Pain Treatment With Cannabidiol in Kidney Transplant Patients in Uruguay. (2018)
- Patterns of medicinal cannabis use, strain analysis, and substitution effect among patients with migraine, headache, arthritis, and chronic pain in a medicinal cannabis cohort. (2018)
- Attenuation of early phase inflammation by cannabidiol prevents pain and nerve damage in rat osteoarthritis. (2017)
- Single and combined effects of Δ9‐tetrahydrocannabinol and cannabidiol in a mouse model of chemotherapy‐induced neuropathic pain. (2017)
- Neurophysiologic correlates of headache pain in subjects with major depressive disorder. (2017)
- Cannabidiol – Delta9-Tetrahydrocannabinol Interactions on Acute Pain and Locomotor Activity (2017)
- Cannabidiol Is a Potential Therapeutic for the Affective-Motivational Dimension of Incision Pain in Rats (2017)
- Low-Dose Cannabidiol Is Safe but Not Effective in the Treatment for Crohn’s Disease, a Randomized Controlled Trial. (2017)
- Cannabidiol-Δ9-tetrahydrocannabinol interactions on acute pain and locomotor activity. (2017)
- Transdermal cannabidiol reduces inflammation and pain‐related behaviours in a rat model of arthritis. (2016)
- Transdermal cannabidiol reduces inflammation and pain‐related behaviors in a rat model of arthritis. (2016)
- A double‐blind, randomized, placebo‐controlled, parallel group study of THC/CBD spray in peripheral neuropathic pain treatment. (2014)
- A Double-blind, Randomized, Placebo-controlled, Parallel-group Study of THC/CBD Oromucosal Spray in Combination With the Existing Treatment Regimen, in the Relief of Central Neuropathic Pain in Patients With Multiple Sclerosis (2013)
- An Open-label Extension Study to Investigate the Long-term Safety and Tolerability of THC/CBD Oromucosal Spray and Oromucosal Thc Spray in Patients With Terminal Cancer-related Pain Refractory to Strong Opioid Analgesics (2013)
- Multicenter, double-blind, randomized, placebo-controlled, parallel-group study of the efficacy, safety, and tolerability of THC: CBD extract and THC extract in patients with intractable cancer-related pain. (2010)
- Antinociceptive effect of the cannabinoid agonist, WIN 55,212‐2, in the orofacial and temporomandibular formalin tests. (2010)
- Cluster attacks responsive to recreational cannabis and dronabinol. (2009)
- Sativex successfully treats neuropathic pain characterised by allodynia: a randomised, double-blind, placebo-controlled clinical trial. (2007)
- Cannabis, pain, and sleep: lessons from therapeutic clinical trials of Sativex®, a cannabis‐based medicine. (2007)
- Oromucosal Delta9-tetrahydrocannabinol/cannabidiol for Neuropathic Pain Associated With Multiple Sclerosis: an Uncontrolled, Open-label, 2-year Extension Trial (2007)
- The Non-psychoactive Cannabis Constituent Cannabidiol Is an Orally Effective Therapeutic Agent in Rat Chronic Inflammatory and Neuropathic Pain (2007)
- Meta-analysis of Cannabis Based Treatments for Neuropathic and Multiple Sclerosis-related Pain (2007)
- The non-psychoactive cannabis constituent cannabidiol is an orally effective therapeutic agent in rat chronic inflammatory and neuropathic pain. (2007)
- Cannabis, pain, and sleep: Lessons from therapeutic clinical trials of sativexρ, a cannabis-based medicine. (2007)
- Cannabis, pain, and sleep: Lessons from therapeutic clinical trials of sativex, a cannabis-based medicine (2007)
- Cannabidiol is an allosteric modulator at mu-and delta-opioid receptors. (2006)
- Sativex®: clinical efficacy and tolerability in the treatment of symptoms of multiple sclerosis and neuropathic pain. (2006)
- Vanilloid TRPV1 Receptor Mediates The Antihyperalgesic Effect Of The Nonpsychoactive Cannabinoid, Cannabidiol, In A Rat Model Of Acute Inflammation (2004)
- Cannabidiol-transdermal delivery and anti-inflammatory effect in a murine model. (2003)
- he cannabinoids: an overview. Therapeutic implications in vomiting and nausea after cancer chemotherapy, in appetite promotion, in multiple sclerosis and in neuroprotection. (2001)

CBD Research: Sleep
Research supports the following effects of CBD for sleep:
- Sedative qualities promote faster sleep onset
- May reduce nightmares
- Regulates the sleep cycle and sleeping patterns
- Alleviates common causes of insomnia
List of research studies involving CBD & sleep:
- A Cross-Sectional Study of Cannabidiol Users. (2018)
- No Acute Effects of Cannabidiol on the Sleep-Wake Cycle of Healthy Subjects: A Randomized, Double-Blind, Placebo-Controlled, Crossover Study. (2018)
- Cannabidiol can improve complex sleep‐related behaviours associated with rapid eye movement sleep behaviour disorder in Parkinson’s disease patients: a case series. (2014)
- Effect of Δ-9-Tetrahydrocannabinol and Cannabidiol on Nocturnal Sleep and Early-Morning Behavior in Young Adults. (2004)
CBD Research: Weight Loss
Research supports the following effects of CBD for weight loss:
- Reduces appetite to curb eating habits
- Alleviates chronic inflammation associated with obesity
- Supports optimal hormone function
- Alleviates digestive disorders & inflammation
- Supports mitochondria function (where sugar & fat is burned for energy)
- May reduce insulin resistance
- Alleviates stress & anxiety
List of research studies involving CBD & weight loss:
- Cannabis exposure associated with weight reduction and β-cell protection in an obese rat model. (2012)
- A pilot study of the effects of cannabis on appetite hormones in HIV-infected adult men. (2012)
- Cannabidiol Attenuates the Appetitive Effects of Δ9-Tetrahydrocannabinol in Humans Smoking Their Chosen Cannabis. (2010)
- Endocannabinoids in the regulation of appetite and body weight (2005)
Why CBD Research is So Important
Since the prohibition on cannabis was lifted in the Western world, there’s been a great deal of confusion as to how to best regulate CBD and other cannabis-based products.
Pharmaceutical companies are in a race to create and patent cannabinoid-based prescription drugs like Epidiolex or Sativex.
On the supply side, botanical extract manufacturers are keen to meet growing market demands, but at the same time must carefully navigate an uncertain regulatory environment.
The convergence of these conflicting interests is a recipe for trouble and raises some important areas of concern:
- What do we really know about the short- and long-term safety of CBD?
- What conditions can CBD be used to support?
- Are there health conditions that could be made worse by CBD?
- How does CBD interact with prescription and non-prescription medications?
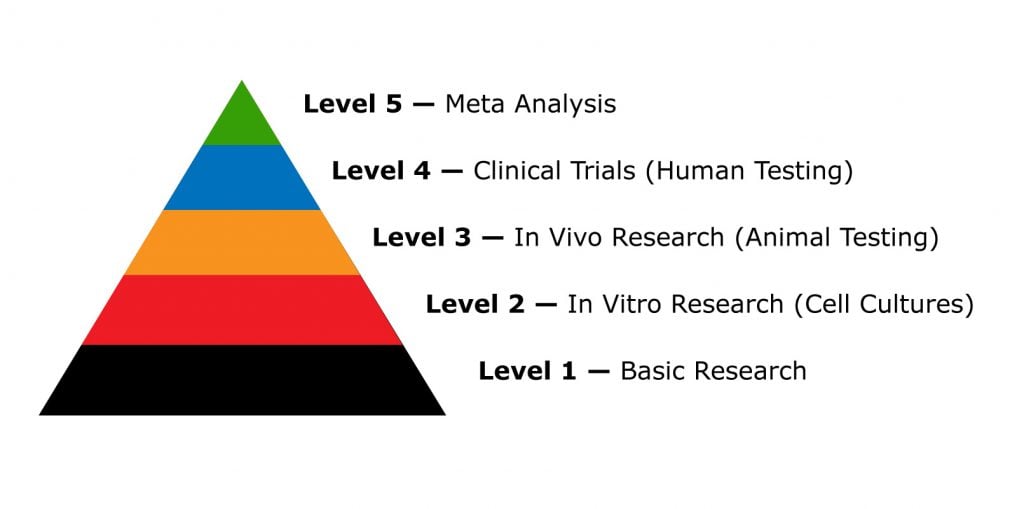
Five Types of CBD Research
As the CBD industry continues to evolve — there’s an endless supply of questions scientists will continue to seek answers for.
Answering these questions will rely on scrutinous scientific exploration. Through different levels of research, CBD can be vetted to the same stringent standards for safety and efficacy as any other drug or botanical extract.
CBD research falls into three main categories:
- Level 1: Basic Research — low-level research to map the structural makeup and chemical properties
- Level 2: In Vitro Studies (using cell cultures) — low-level research; begins to explore the toxicity of a substance in live cells and tissues
- Level 3: In Vivo Studies (using real living organisms) — higher-level research involving living non-human animals
- Level 4: Clinical Trials — conducted on real humans in 4 different stages (I, II, III, and IV)
- Level 5: Meta-analysis — a review of published preclinical and clinical research; provides the highest level of evidence possible by pooling the data from similar studies.
So, how does the scientific process work, and what are the methods and stages of research that will ultimately tell us everything we need to know about the benefits and risks of CBD?
Let’s take a closer look at the different levels of scientific research for studying CBD.
Here we provide a glimpse into the painstaking process behind the splashy headlines that appear on an almost daily basis about CBD being nature’s cure for everything from acne to cancer.

Level 1: Basic Research — Is CBD Safe?
CBD is widely regarded as being safe and non-toxic and that’s great news. But, is CBD completely free of any side effects?
This is what basic research studies are designed to explore. Rather than looking at specific benefits of a compound, they assess how much of the compound is needed to produce toxic effects on the body and explore the chemical makeup of CBD or other cannabis extracts.
A common method of assessing toxicity is to give increasing doses of a compound (like CBD) to rats until 50% of the sample population dies. This gives us the LD50 of a compound (lethal dose for 50% of the population).
Careful observation is noted on how the rats die, which gives us a clue as to the toxic side-effects of the compound.
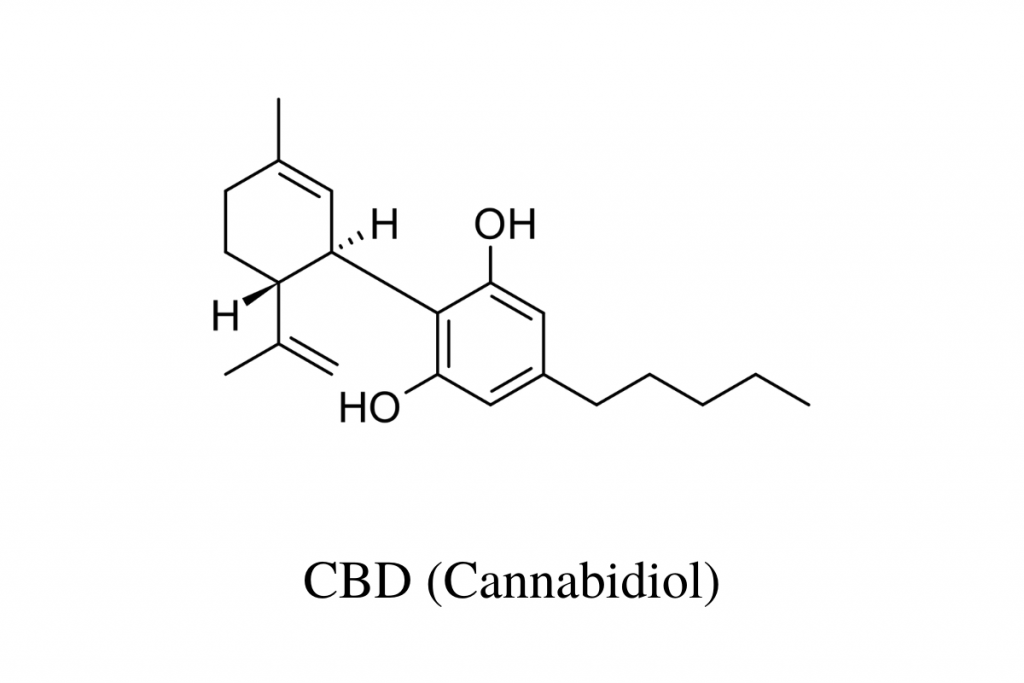
During the first phases of basic research, the structural, molecular, and chemical properties of a substance are determined. This early research tells us that CBD is soluble in fat but not in water and that its molecular formula is C21H30O2.
It’s because of this research that we know CBD looks like this:
Examples of structural property studies involving CBD
| Study Topic | Article Authors | Level of Study |
| The Structure of Cannabidiol | Mechoulam et al., 1963 | 1 |
| Separating Cannabinoids | Lerner., 1963 | 1 |
| Identification of CBD Sub-Groups | Harvey., 1976 | 1 |

Level 2: In Vitro Studies — Testing On Living Cells
Once the structural and chemical properties of CBD are measured and cataloged, researchers introduce controlled amounts of the compound to cell and tissue cultures.
Tissue cultures consist of living cells that are grown in test tubes and Petri dishes (in vitro, literally translated, means “in glass”). The goal of this research is to begin to find out how it will interact with living cells using highly controlled environments.
The in vitro process allows scientists to create precisely controlled environments using a single cell or tissue type, in which all variables can be controlled.
In this phase of research, it’s possible to design an experiment that asks and answers a single question about CBD — which is not possible in a living human with many systems communicating and influencing each other at all times.
Examples of In Vitro CBD Studies
| Study Topic | Article Authors | Level of Study |
| CBD & Stroke | Hind et al., 2016 | 2 |
| CBD & Inflammatory Bowel Disease | Harvey et al., 2014 | 2 |
| CBD & Cancer Cells | Ramer et al., 2010 | 2 |

Level 3: In Vivo Studies — Testing On Live Animals
The next level of research is in vivo research — which is conducted on live animals and allows scientists to obtain more accurate information about the effectiveness, safety, and toxicity of the substance. By using real living systems, researchers can test how a compound will affect the organism as a whole. In vivo testing can compare effects across different species. Usually, mammals are chosen for in vivo medical research since their physiology is most similar to ours.
Gradually, the research is advanced from rodent species (mice, rats, rabbits, etc.) to larger species (cats, dogs, non-human primates). This can reveal how response differs from one species to another and can also help researchers narrow down a safe starting dose when it comes time to test on humans.
With this information, we can start to understand how CBD is likely to travel into and through the body, and how much the body can safely handle. This information is used as a basis for the next stages of research that look closer at the actual interaction CBD has on the body.
In vivo research also determines:
- How CBD travels through the body — how quickly and efficiently it is absorbed into the bloodstream
- How CBD is broken down — whether this is done in the kidneys, liver, or elsewhere in the body
- How safe or toxic CBD and its metabolites are — sometimes a compound is found safe through in vitro research but shows toxic effects when given to live animals. For example, currently there is no known LD50 for CBD in humans; however, a 2011 study found doses of 200-300mg/kg to be lethal in some rhesus monkeys.
- How the compound is excreted from the body — compounds may leave the body through the urine, feces, or even through the skin
- The effects of CBD for certain conditions or symptoms — using animals with health issues, we can test CBD to see if it improves the condition or makes it worse
A potential limitation of in vivo research on CBD is that each species contains different receptor counts. Although all mammals have endocannabinoid systems, the densities and distributions of endocannabinoid receptors can vary a lot from one species to the next — therefore changing the effects of CBD.
For example, rats and rhesus monkeys have higher cannabinoid receptor densities than humans in some parts of their brains and lower density in other parts. This makes it difficult to extrapolate how CBD will act in a human compared to an experimental animal.
Currently, the FDA and associated regulatory agencies are requiring researchers to provide data to substantiate their choice of animal species for their studies. But even with these precautions in place, it’s important to keep in mind that drugs that prove safe and effective in animal testing might have different effects on humans.
Examples of In Vivo CBD Research
| Study Topic | Article Authors | Level of Study |
| CBD for Wound Healing | Klein et al., 2018 | 3 |
| CBD for Fear & Anxiety | Norris et al., 2016 | 3 |
| CBD for Epilepsy | Jones et al., 2010 | 3 |

Level 4: Clinical Research — Testing On Humans
Once sufficient in vitro and in vivo research has been conducted to establish that CBD might be reasonably effective and safe for a particular purpose clinical studies, i.e. experiments on human volunteers, can begin.
Clinical research is considered the highest level of experimental research because it’s done directly on humans. These studies can be used to confirm the pharmacokinetics from in vivo studies and test whether the benefits in laboratory animals can be replicated in humans.
Clinical trials may be used to assess the following:
- The pathway CBD takes through the body, from absorption to distribution to elimination
- The clinical effects of CBD (ie. its health benefits)The absence or presence of side-effects
- The optimal dose needed for CBD to be used effectively in humans
The quality of clinical trials can vary widely, and there are some key elements to consider when assessing the quality of a particular trial.
Placebo-Control
A placebo control is a critical component of a clinical trial to ensure the results of the study weren’t caused by the participants’ expectations. To apply a placebo control, a sham treatment is given to one group (an inactive compound that looks like CBD but isn’t).
This procedure is designed to eliminate the placebo effect — where symptoms may improve after a patient believes they were using a medication, even if there were no active compounds in the treatment. The placebo effect has been found to occur in about 40% of study participants.
Randomization
To make sure there are no patterns in the treatment group or placebo control groups that may affect results, statistical randomization is used to separate the participants.
This process assures that the participants are equally distributed to each arm of the study.
Randomization prevents all participants with a particular characteristic, such as age, sex, or a genetic risk factor to be placed in the same group. It also prevents conscious or subconscious researcher bias from influencing the experiment, such as placing all of the participants most likely to have a good (or bad) response in the same group.
Blinding
Blinding is used to make it unknown to the study participants and researchers which participants are getting a dose of CBD or placebo. This eliminates bias by not revealing which treatment is being administered.
There are two categories of blinding:
Single-blind — the participant is not informed of which treatment they are receiving
This prevents the participants’ preconceived ideas or expectations from influencing their responses. Single blinding can help eliminate the placebo effect.
Also, many studies rely on subjective reports by participants about their symptoms and overall experience. Single blinding eliminates these potential biases. If they think they’re getting the treatment, they may answer the questions differently than if they knew they were taking the placebo. So it’s best to keep this part a mystery until the trial is over.
Double-blind — both the participant and the researcher don’t know which treatment is being administered to which participant. This prevents the researcher from consciously or subconsciously behaving differently toward participants in ways that could influence the results of the study.
The best clinical trials use double-blinding in their research model.
Different Levels of Clinical Trials
A) Phase I Trials
Phase I trials are the first studies in which a drug is given to a small group of people (up to 80). In this phase, the best route of administration, i.e. by mouth, inhaled, topical, injected into the bloodstream, etc. is determined, as well as ideal dosing levels and frequency.
If this study proves safe and shows improvement, the study can move on to phase II.
B) Phase II Trials
Phase II clinical trials obtain further safety information and begin to collect data on effectiveness. These studies involve larger numbers of participants — usually between 100 to 300.
C) Phase III Trials
Phase III clinical trials compare the new drug to a drug that is commonly used as the standard of care for a particular condition. These studies use large numbers of participants (from 300 to 3,000 or more).
Once a drug is approved by the FDA, the manufacturer may elect or they may be required by regulatory agencies to conduct the fourth phase of trials.
D) Phase IV Trials
Phase IV clinical trials determine long-term side effects and the risk-to-benefit ratio for someone who may need to use the drug ongoing over a long period of time. These studies use the greatest number of people, with participants potentially numbering in the thousands [13]. They also tend to cover several months, or even years to collect long-term health data.
E) Epidemiological Research
Once a CBD-based drug has been FDA-approved it can be included in epidemiological research. Epidemiology seeks to understand the frequency and distribution patterns of a disease or health condition.
Part of epidemiology involves studying the effects of interventions, which can include eliminating exposure to environmental toxins and pollutants, changing lifestyle behaviors that predispose people to develop certain conditions, and also the effects of drugs or other substances, such as CBD, on the distribution of a particular disease within the population.
To better understand the value of clinical CBD studies, the types of information they provide, and their drawbacks, let’s take a close look at a few examples.
Examples of Clinical Trials Involving CBD
| Study Topic | Article Authors | Level of Study |
| CBD for Crohn’s Disease | Naftali et al., 2017 | 4 |
| CBD for Anxiety and Insomnia | Shannon et al., 2019 | 4 |
| CBD for Healthy People | Taylor et al., 2018 | 4 |
Level 5: Meta-Analysis — Comparing Data From Multiple Studies
A meta-analysis is a type of systematic review — a study of studies — in which the data from multiple studies on a particular aspect of CBD are compiled and evaluated together. A meta-analysis can include either pre-clinical or clinical studies.
The higher the level of studies used in the meta-analysis the stronger the evidence will be for a particular effect. For example, a meta-analysis that uses double-blind, placebo-controlled, randomized human trials will produce stronger evidence than a meta-analysis of animal studies.
Examples of Meta-Analysis Involving CBD
| Study Topic | Article Authors | Level of Study |
| CBD for Epilepsy | de Carvalho Reis et al., 2019 | 5 |
| CBD for Stroke | England et al., 2015 | 5 |
| Effects of CBD on the Circulatory System | Wade et al., 2010 | 5 |
Key Takeaways: What’s The Future of CBD Research?
Cannabis research was curtailed for nearly 80 years due to prohibition. Now scientists are working hard to fill in the gaps but at the same time the regulatory landscape is uncertain and the information available to CBD consumers can be confusing.
Scientists are using different research models to answer some of the biggest questions we have around the use of CBD:
- How safe is CBD?
- What can CBD be used to help?
- Can CBD worsen any conditions?
- How does CBD interact with medications?
Knowing some of the how’s and why’s of scientific research can help you interpret the latest headlines about CBD so you can be an informed consumer.
Scientific medical studies can be divided into five different categories:
- Level 1: Basic Research — early research for assessing the molecular structure and chemical properties
- Level 2: In-Vivo Research — highly-controlled research for assessing beneficial and toxic effects of CBD on isolated cells and tissues grown in test tubes
- Level 3: In-Vitro Research — real testing of CBD on living systems to assess safety and effectiveness
- Level 4: Clinical trials — testing on humans to assess dose, efficacy, and comparison of CBD with other compounds
- Level 5: Meta-Analysis — continued data analysis of multiple similar studies on a particular effect profile
Through continued research, building up from basic research all the way up to large-scale meta-analysis, we can seek a deeper understanding of how CBD works, and how to use it both safely and effectively for a wide range of different symptoms and health conditions.